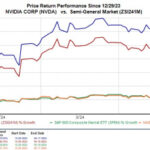
Shares of Sarepta Therapeutics (SRPT) are up about 38% in after-hours trade after the company announced that the FDA will allow it to file for a New Drug Application — NDA — for Eteplirsen. Eteplirsen is the company’s drug to treat Duchenne muscular dystrophy — DMD. Patients who suffer from DMD have limited muscle movement if any movement at all. This is because this disease causes progressive muscle degeneration which leads to less movement function and even loss of the ability to walk.
Eteplirsen is an RNA drug that is supposed to attempt to skip an exon in the protein in hopes of allowing the dystrophin to be produced properly in these patients. Dystrophin levels in DMD patients slowly deplete, causing the many functional problems including muscle weakness. Some parts of the application to the FDA will be submitted this week, but the rest of the application will be completed by mid year 2015.
If the filing goes well it is possible that the FDA will set up a review panel to look at the efficacy and safety of Eteplirsen in DMD patients. Another competing drug from Biomarin Pharmaceuticals (BMRN), known as drisapersen, also treats DMD. It’s up in the air now on how both drugs will do with the FDA deciding, but ultimately we hope that one of these drugs are approved as these patients desperately need these treatments. With these treatments patients will have more muscle movement and may even be able to walk a certain distance.