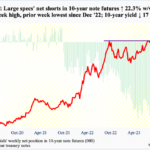
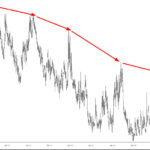
Top Pick of the Day: CELG
Celgene Corporation (CELG) announced that it has agreed to acquire a stake in BeiGene. The deal has valued BeiGene stock at $4.58 apiece. The company will buy 5.9 percent of the total stock, which would be 32.7 million shares. BeiGene is set to receive $263 million in upfront license fee. It is also entitled to receive up to $980 million in development and other milestone payments related to the tumor cancer treatment. According to BeiGene, more than 500 patients have been dosed with its BGB-A317 advanced clinical-stage investigational tumor cancer treatment.
The company stock is currently trading close to its 52 weeks high of $135.18. It has gained over 14 percent of its value this year so far, while its 12 months gain stands at 26 percent.
Focus Ticker: TNXP
Tonix Pharmaceuticals Holding Corp (TNXP) announced that the FDA has conditionally accepted the proposed trade name Tonmya for TNX-102 SL* (cyclobenzaprine HCl sublingual tablets) for the management of posttraumatic stress disorder (PTSD). The U.S. Patent and Trademark Office has already granted the federal registration of the Tonmya mark. However, the company has withdrawn a request with the FDA for review of Tonmya as the proposed name for TNX-102 SL for the management of fibromyalgia.
The company stock is currently trading close to its 52 weeks low of $3.30. It has lost 79 percent of its value in the past 12 months while its Year to Date loss stands at 7 percent.
Sector News
Inotek Pharmaceuticals (ITEK) announced disappointing top-line results from a mid-line trial for glaucoma drug trabodenoson used in combination with the old standard prostaglandin latanoprost. The study resulted in a 1.2 mmHg intraocular pressure improvement over latanoprost alone. However, its follow up four weeks dosing at night did not show any meaningful clinical advantage between the combo and the solo therapy.
Biogen (BIIB) announced that the European Medicines Agency has restricted the use of its Zinbryta until the completion of a liver safety investigation. The new safety review was launched pursuant to liver failure death of a patient in an ongoing study and four cases of severe liver damage.